Program Snapshot
The NIH Common Fund’s Somatic Cell Genome Editing (SCGE) program aims to reduce the burden of diseases caused by genetic changes. Genome editing technologies present an exciting prospect for treatments and possibly even cure for these diseases. During its first 5-year phase (FY18-FY23), SCGE developed quality tools to perform and assess effective genome editing tools in non-reproductive (“somatic”) cells of the body. In its second phase (FY23-27), SCGE will accelerate the translation of genome editing therapies into the clinic.
SCGE Phase 1
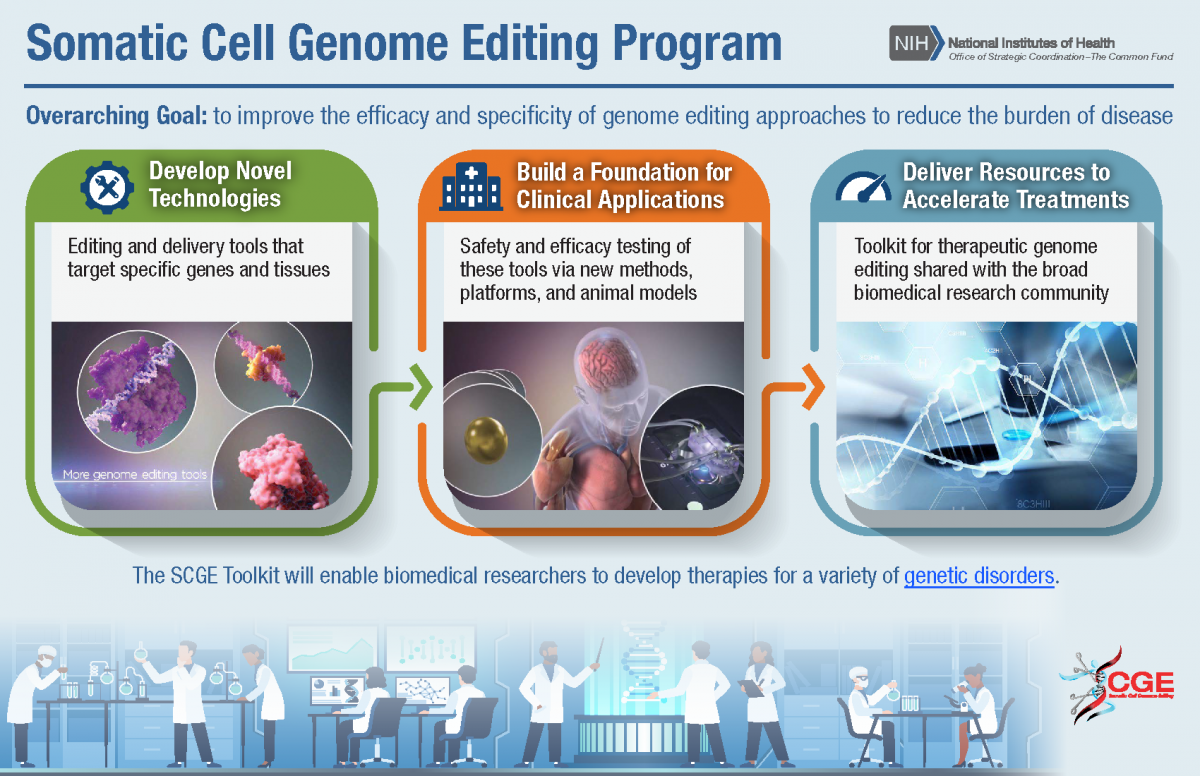
SGCE worked to improve the efficacy and specificity of genome editing approaches. The SCGE program developed new methods and improved systems that delivered genome editing machinery into various tissues with greater specificity, including tissues that present an unmet need and that are harder to reach such as the brain, ear, heart, and lung. SCGE made significant discoveries of new or optimized editors that edit target genomes with improved efficacy and novel functionality, including a prime editor that could correct up to 89% of known genetic variants associated with human diseases. The SCGE program developed new methods to assess unintended biological effects, such as high-throughput technologies that quickly identify high specific target sites and follow genome-wide activity of editors over time. Through the development of better animal models, the SCGE program has tested and validated a number of genome editing tools and delivery systems. The program has developed a genome editing toolkit that broadly disseminates program tools and learnings, opening this space to a wider range of the biomedical research community.
Learn more about the goals of SCGE Phase 1
An audio described version of this video is also available.
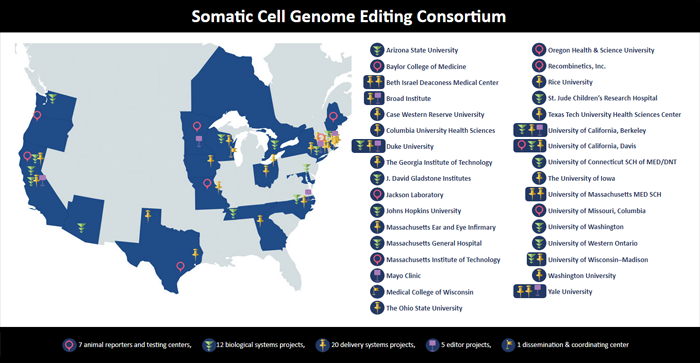
SCGE Phase 1 Consortium
Click the image below to view the interactive Somatic Cell Genome Editing Consortium map.
SCGE Phase 2
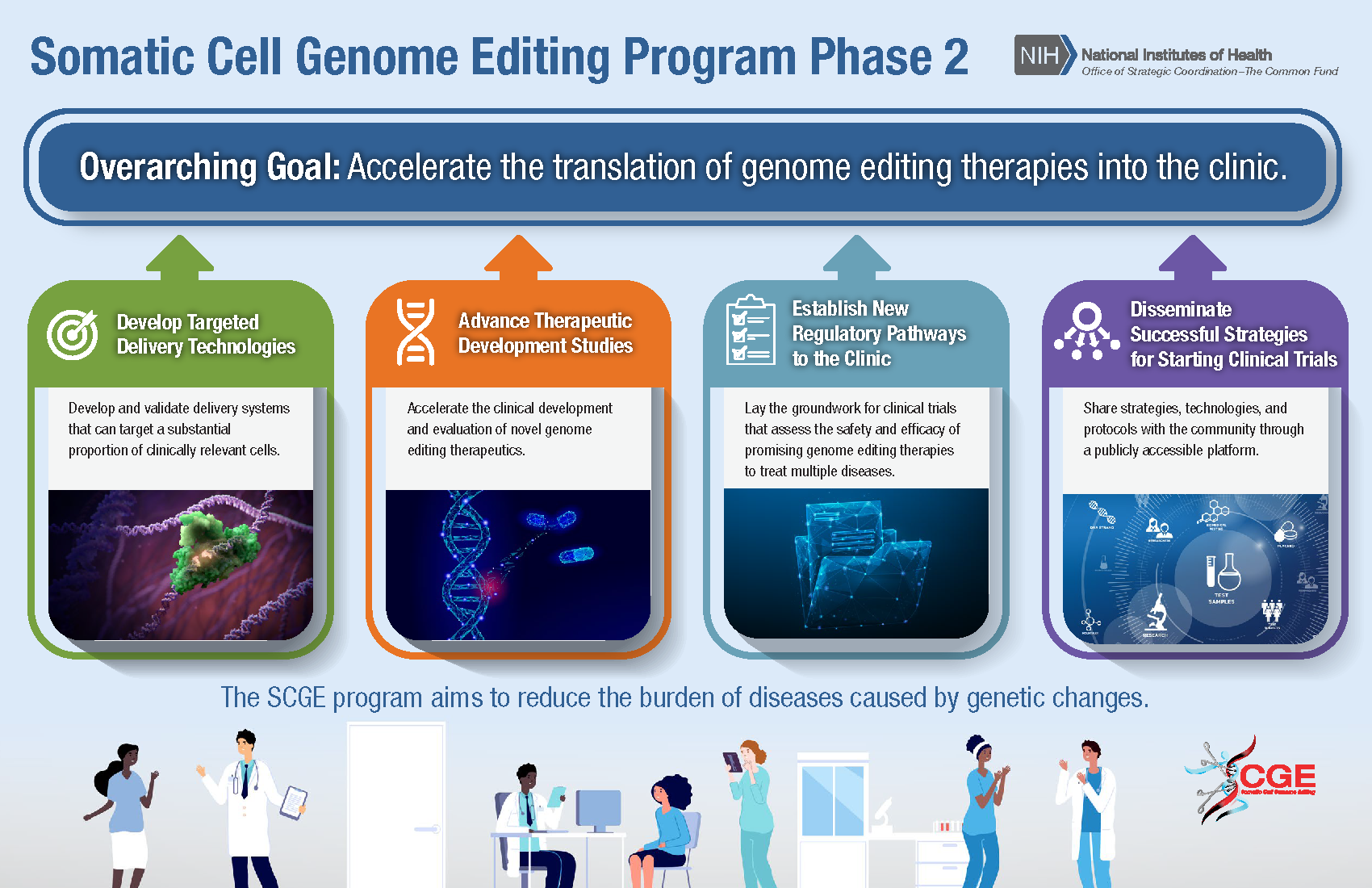 SCGE will accelerate the translation of genome editing therapies into the clinic by developing targeted delivery technologies; advancing clinical development and evaluation of novel genome editing therapeutics; establishing new regulatory pathways to lay the groundwork for clinical trials that assess the safety and efficacy of promising genome editing therapies to treat multiple diseases; and disseminating successful strategies for starting clinical trials through a publicly accessible platform.
SCGE will accelerate the translation of genome editing therapies into the clinic by developing targeted delivery technologies; advancing clinical development and evaluation of novel genome editing therapeutics; establishing new regulatory pathways to lay the groundwork for clinical trials that assess the safety and efficacy of promising genome editing therapies to treat multiple diseases; and disseminating successful strategies for starting clinical trials through a publicly accessible platform.
SCGE phase 2 will consist of four initiatives:
- Developing technologies and assays for safety and efficacy studies
- Optimizing genome editing-based therapeutic leads to support advancement towards clinical trials
- Supporting novel genome editing clinical trials for more than one disease
- Fostering collaboration and share new technologies and protocols with the public and research community
Existing gene editing technologies have great potential but are not able to deliver gene editing tools to many target tissues and cell types in sufficient quantities, which hinders clinical application. To realize the promise of genome editing for treating many genetic diseases, SCGE launched the multi-phase TARGETED (Targeted Genome Editor Delivery) Challenge to improve the current state of in vivo delivery technologies for genome editors in two Target Areas: 1. Programmable Delivery System for Gene Editing, and 2. Crossing the Blood-Brain Barrier. The TARGETED Challenge will help improve these technologies and speed their translation from the lab into the clinic.
Follow SCGE
Subscribe to receive updates from SCGE. 
News & Announcements
- NIH launches the second phase of the TARGETED Challenge to revolutionize in vivo delivery technologies for genome editors
- NIH announces the Phase 1 winners of the TARGETED Challenge spanning two target areas: Programmable Delivery System for Gene Editing & Crossing the Blood-Brain Barrier
- New Notice of Funding Opportunity RFA-RM-24-007: Technologies and Assays for Therapeutic Genome Editing INDs (U01 Clinical Trial Not Allowed)
- SCGE launches second phase to accelerate the translation of genome editing approaches from the lab to the clinic
- NIH Announces Launch of the TARGETED Challenge
- NIH approves a second phase of the SCGE program
- A new gene editing tool has the potential to correct most disease-causing DNA glitches (Journal Access Required)
- SCGE Announces Additional Awards to Advance Genome Editing
- A Nano-Sized Solution for Safe and Efficient Gene Editing SCGE awardees, Dr. Shaoqin Sarah Gong and Dr. Krishanu Saha, developed nanocapsules to deliver gene editing agents to different target cells in the body.
- NIH to Launch Genome Editing Research Program
- SCGE Program Announces First Awards to Improve Genome Editing Techniques
Science Highlights
SCGE Phase II
NIH approves a second phase of the SCGE program
- September 17, 2021 - NIH Council of Councils presentation (at time 1:38)
- September 17, 2021 - NIH Council of Councils slides
NIH issued new funding opportunities to support the second phase of the SCGE program. A pre-application webinar was held for potential applicants on May 4, 2022. Click here to access the webinar slides.
SCGE Dissemination and Coordinating Center
Visit the SCGE Dissemination and Coordinating Center website for more information about the program.
Program Background
- Genome Editing to 'Re-write" Wrongs - a commentary on the rationale behind the SCGE program.
- SCGE Overview at NIH's Rare Disease Day 2018
- September 1, 2017 - NIH Council of Councils presentation (at time 2:53)
- September 1, 2017 - NIH Council of Councils slides
What is Genome Editing?
Visit NHGRI's Genome Editing website to learn more about this technology.