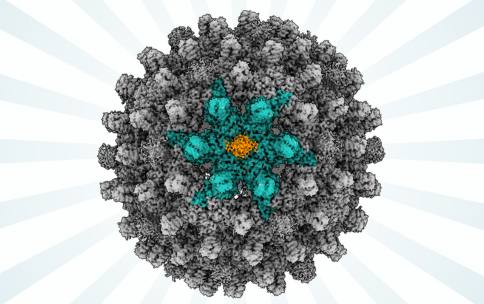
Infection of hepatitis B virus (HBV) is responsible for more than 800,000 deaths per year around the world. People infected with the virus can eventually develop liver disease and liver cancer. Although vaccines are available to prevent initial infection with HBV, there is no effective treatment that can cure someone once they become infected with HBV. Viruses enter the nucleus of the cell to replicate and cause damage that makes us sick. Viruses like HBV have an outer shell of proteins that allow the virus to enter the host cell and nucleus. In the case of HBV, the details of how this sphere of proteins interacts with the host cell and the nucleus of the host cell, is not entirely known. Visualizing the protein shell of HBV is an important strategy to design anti-viral drugs that could treat or cure infection. The technique of cryogenic electron microscopy (CryoEM) enabled by resources at CryoEM Centers supported by the Transformative CryoEM Common Fund Program have visualized details of the structure of HBV proteins to help researchers better understand the molecular details of HBV interactions.
In a study published in Science Advances, a team of researchers leveraged the National Center for CryoEM Access and Training (NCCAT), one of three centers supported by the CryoEM Common Fund Program, to visualize molecular details of HBV. Led by Dr. Gino Cingolani at the University of Alabama at Birmingham, researchers revealed that two proteins from the host cell were necessary for enabling viral entry into the nucleus, detailing a previously unknown mechanism as to how HBV hijacks the host cell’s own machinery to reproduce. This study was performed on isolated proteins, the researchers did not observe these processes in living human cells, so further work remains to understand in even finer detail how these proteins are involved in the uptake of HBV into a host-cell nucleus.
The NIH Common Fund’s Transformative CryoEM Program broadens access to the technique of cryogenic electron microscopy for biomedical researchers to better understand and eventually treat diseases.
Reference: Structural basis for nuclear import of hepatitis B virus (HBV) nucleocapsid core. Yang R, Ko YH, Li F, Lokareddy RK, Hou CD, Kim C, Klein S, Antolínez S, Marín JF, Pérez-Segura C, Jarrold MF, Zlotnick A, Hadden-Perilla JA, Cingolani G Science Advances. 2024 January;10(2). DOI: 10.1126/sciadv.adi7606.


