Science Highlights Archives
Pharmaceutical Gift Giving Influences Physician Prescription Practices
 Peter Bearman, a 2007 Pioneer, examined how state restrictions on pharmaceutical marketing influence how physicians prescribe their drugs. Bearman and colleague found the uptake of new costly medications was significantly lower in states with regulations on pharmaceutical marketing than in states with no restrictions. States with gift bans had a reduction of 39% to 83% in pharmaceutical sales of four new psychotropic drugs. In restricted markets, peer influence replaced pharmaceutical marketing for informing physicians of new, beneficial drugs with less bias. They suggest gift bans coupled with mandatory public disclosure of exempt items are an effective way to reduce the prescription of new medications and help manage conflicts of interest.
Peter Bearman, a 2007 Pioneer, examined how state restrictions on pharmaceutical marketing influence how physicians prescribe their drugs. Bearman and colleague found the uptake of new costly medications was significantly lower in states with regulations on pharmaceutical marketing than in states with no restrictions. States with gift bans had a reduction of 39% to 83% in pharmaceutical sales of four new psychotropic drugs. In restricted markets, peer influence replaced pharmaceutical marketing for informing physicians of new, beneficial drugs with less bias. They suggest gift bans coupled with mandatory public disclosure of exempt items are an effective way to reduce the prescription of new medications and help manage conflicts of interest.
Reference: Gifts and Influence: Conflict of Interest Policies and Prescribing of Psychotropic Medications in the United States. King M, Bearman, PS. Social Science Medicine. 2017 Jan;172:153-162.
New Technique Blasts Circulating Cancer Cells in the Blood
 Cancer cells can spread to new areas of the body, often through the blood, and causes nearly 90% of cancer-related deaths. Numerous lives would be saved if cancer cells that break away from a primary tumor could be detected and destroyed in the blood before the cells spread and form new tumors. Lihong Wang, a 2012 Pioneer and 2013 Transformative Researcher, has developed a method that promises to do just that. Using the unique optical characteristics of skin cancer-causing melanoma cells, Wang and his team developed lasers that can detect and target melanoma cells circulating in the blood and vaporize them without harming surrounding tissue. Preliminary studies are encouraging and provide hope that the technique may be useful in preventing the spread of cancer throughout the body.
Cancer cells can spread to new areas of the body, often through the blood, and causes nearly 90% of cancer-related deaths. Numerous lives would be saved if cancer cells that break away from a primary tumor could be detected and destroyed in the blood before the cells spread and form new tumors. Lihong Wang, a 2012 Pioneer and 2013 Transformative Researcher, has developed a method that promises to do just that. Using the unique optical characteristics of skin cancer-causing melanoma cells, Wang and his team developed lasers that can detect and target melanoma cells circulating in the blood and vaporize them without harming surrounding tissue. Preliminary studies are encouraging and provide hope that the technique may be useful in preventing the spread of cancer throughout the body.
Reference: In Vivo Label-Free Photoacoustic Flow Cytography and On-the-Spot Laser Killing of Single Circulating Melanoma Cells. He Y et al. Scientific Reports. 2016 Dec 21;6:39616.
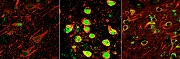
Visualizing RNA using Expansion Microscopy
 RNA molecules act as messengers, carrying copies of genetic instructions from the DNA throughout the cell and are crucial for protein synthesis and proper cell functioning. Arjun Raj (a 2011 New Innovator) and Edward Boyden (a 2007 New Innovator, 2012 and 2013 Transformative Researcher, and 2013 Pioneer) describe a new technique that can image RNA in cells and tissue by using “expansion microscopy,” a method where samples are physically enlarged with an expandable polymer, making high resolution imaging possible with common laboratory microscopes. Knowing the distribution of RNA inside cells will enable scientists to better understand how cells control gene expression and could aid study of diseases caused by the improper movement of RNA.
RNA molecules act as messengers, carrying copies of genetic instructions from the DNA throughout the cell and are crucial for protein synthesis and proper cell functioning. Arjun Raj (a 2011 New Innovator) and Edward Boyden (a 2007 New Innovator, 2012 and 2013 Transformative Researcher, and 2013 Pioneer) describe a new technique that can image RNA in cells and tissue by using “expansion microscopy,” a method where samples are physically enlarged with an expandable polymer, making high resolution imaging possible with common laboratory microscopes. Knowing the distribution of RNA inside cells will enable scientists to better understand how cells control gene expression and could aid study of diseases caused by the improper movement of RNA.
- Nanoscale Imaging of RNA with Expansion Microscopy. Chen F et al. Nat Methods. 2016 Aug 13;13(8):679-84.
- In the News: Nanoscale Microscopy Technique Allows Scientists to Pinpoint RNA Molecules in the Brain
Stuttering Mice Help Study Human Disorder
 Timothy Holy, a 2009 Pioneer, created mice mimicking stuttering in humans. Mice carrying the same mutations linked to stuttering in humans show changes in the patterns of their calls in timing and temporal vocalization. The mutant mice otherwise showed no other symptoms or impairments, similar to human stutterers. The mouse model provides opportunities for new research in stuttering and may ultimately lead to treatments for human stutter sufferers.
Timothy Holy, a 2009 Pioneer, created mice mimicking stuttering in humans. Mice carrying the same mutations linked to stuttering in humans show changes in the patterns of their calls in timing and temporal vocalization. The mutant mice otherwise showed no other symptoms or impairments, similar to human stutterers. The mouse model provides opportunities for new research in stuttering and may ultimately lead to treatments for human stutter sufferers.
- A Mutation Associated with Stuttering Alters Mouse Pup Ultrasonic Vocalizations. Barnes TD, Wozniak DF, Gutierrez J, Han TU, Drayna D, Holy TE. Curr Biol. 2016 Apr 13;pii: S0960-9822(16)30179-8.
- In the News: ‘Stuttering’ Mice May Help Unravel Mystery of Human Speech Disorder
- In the News: Stuttering Mouse Experiment Sheds Light on Common Human Speech Disorder
- In the News: Stuttering Mice Could Help Stuttering Humans
- In the News: Stuttering Mice Could Reveal New Clues About the Speech Disorder
- In the News: Stuttering Mice Could Help Scientists Unlock Secrets Of Human Speech Disorders
- In the News: Stuttering Mice Could Be Key to Fixing Human Speech Disorder
- In the News: Researchers Genetically Engineer Mice to Stutter, Laying Basis for Model of the Human Speech Disorder
- In the News: Mice Are Being Bred with Stutters to Help Study How the Speech Impediment Develops
Controlling Memories
 Lorna Role, a 2010 Pioneer, examined the effect of acetylcholine in the amygdala, a region of the brain involved in emotional memories, and the strength of those memories. Using a fear-based memory model in mice, Role and colleagues tested the underlying mechanism of memory using optogenetics, which can control living cells using light. They found when an increased amount of acetylcholine is released in the amygdala during the formation of a traumatic memory, it strengthens the memory and makes it last more than twice as long as normal. When acetylcholine is decreased in the amygdala during a traumatic experience, the memory was wiped out. The ultimate goal of the research is to enhance or diminish the strength of specific memories to help in disease treatment or post-traumatic stress disorder.
Lorna Role, a 2010 Pioneer, examined the effect of acetylcholine in the amygdala, a region of the brain involved in emotional memories, and the strength of those memories. Using a fear-based memory model in mice, Role and colleagues tested the underlying mechanism of memory using optogenetics, which can control living cells using light. They found when an increased amount of acetylcholine is released in the amygdala during the formation of a traumatic memory, it strengthens the memory and makes it last more than twice as long as normal. When acetylcholine is decreased in the amygdala during a traumatic experience, the memory was wiped out. The ultimate goal of the research is to enhance or diminish the strength of specific memories to help in disease treatment or post-traumatic stress disorder.
Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Jiang L et al. Neuron. 2016 Jun 1;90(5):1057-70.
Treating Mitochondrial Disease with Hypoxia
 Vamsi Mootha (2011 Transformative Researcher) and Feng Zhang (2010 and 2015 Transformative Researcher and 2012 Pioneer) published a paper on using low levels of oxygen to treat mitochondrial diseases, which are debilitating and largely untreatable. The mitochondrial respiratory chain uses oxygen to generate cellular energy, and impairments to it can cause blindness, deafness, and disease in the brain, heart, muscles, stomach, blood, liver, and kidney. However, endogenous mechanisms may exist to help cells cope with mitochondrial defects. Mootha et al. identified the hypoxia response, a mechanism that helps cells adapt to low oxygen levels, as an effective treatment to mitochondrial dysfunction. Zebrafish and mouse models of the mitochondrial disease Leigh syndrome showed fewer symptoms and had a dramatically longer life span when raised in a low oxygen environment. Chronic hypoxia increased survival, body weight, body temperature, behavior, neuropathology, and disease in the mouse model. While further studies are needed to assess the safety of using hypoxic conditions to treat human diseases associated with mitochondrial dysfunction, it may be that the body’s naturally evolved response to low oxygen may be protective against mitochondrial disease.
Vamsi Mootha (2011 Transformative Researcher) and Feng Zhang (2010 and 2015 Transformative Researcher and 2012 Pioneer) published a paper on using low levels of oxygen to treat mitochondrial diseases, which are debilitating and largely untreatable. The mitochondrial respiratory chain uses oxygen to generate cellular energy, and impairments to it can cause blindness, deafness, and disease in the brain, heart, muscles, stomach, blood, liver, and kidney. However, endogenous mechanisms may exist to help cells cope with mitochondrial defects. Mootha et al. identified the hypoxia response, a mechanism that helps cells adapt to low oxygen levels, as an effective treatment to mitochondrial dysfunction. Zebrafish and mouse models of the mitochondrial disease Leigh syndrome showed fewer symptoms and had a dramatically longer life span when raised in a low oxygen environment. Chronic hypoxia increased survival, body weight, body temperature, behavior, neuropathology, and disease in the mouse model. While further studies are needed to assess the safety of using hypoxic conditions to treat human diseases associated with mitochondrial dysfunction, it may be that the body’s naturally evolved response to low oxygen may be protective against mitochondrial disease.
Hypoxia as a therapy for mitochondrial disease. Jain IH et al. Science. 2016 Apr 1;352(6281):54-61.
Uncovering RNA Structure
 Pehr Harbury, a 2005 Pioneer, developed a method of viewing the dynamic behavior of RNA in solution. Using X-ray scattering interferometry, Harbury studied the RNA kink-turn motif and its complexes in solution to demonstrate the how the method can reveal structural and ensemble properties of RNAs and RNA-protein complexes. Such information is necessary to understand, predict, and engineer the behavior and function of RNAs and their protein complexes.
Pehr Harbury, a 2005 Pioneer, developed a method of viewing the dynamic behavior of RNA in solution. Using X-ray scattering interferometry, Harbury studied the RNA kink-turn motif and its complexes in solution to demonstrate the how the method can reveal structural and ensemble properties of RNAs and RNA-protein complexes. Such information is necessary to understand, predict, and engineer the behavior and function of RNAs and their protein complexes.
The Solution Structural Ensembles of RNA Kink-Turn Motifs and Their Protein Complexes. Shi X, Huang L, Lilley DM, Harbury PB, Herschlag D. Nat Chem Biol. 2016 Mar;12(3):146-52.
Tracing Free-Floating DNA Back to Its Source
 Jay Shendure, a 2013 Pioneer, developed a method capable of identifying the source of free-floating DNA circulating in blood plasma. DNA wraps around nucleosomes, and nucleosome positioning varies between cell types. By sequencing the cell-free DNA, a map of DNA fragments creates a footprint of transcription factors, which can be compared to nuclear architecture, gene structure, and expression in a variety of cells to suggest where the cell originated. The method opens the possibility of being able to identify and trace the source of cancers and improve the diagnosis, treatment, and management of a vast array of health conditions all with a simple blood draw.
Jay Shendure, a 2013 Pioneer, developed a method capable of identifying the source of free-floating DNA circulating in blood plasma. DNA wraps around nucleosomes, and nucleosome positioning varies between cell types. By sequencing the cell-free DNA, a map of DNA fragments creates a footprint of transcription factors, which can be compared to nuclear architecture, gene structure, and expression in a variety of cells to suggest where the cell originated. The method opens the possibility of being able to identify and trace the source of cancers and improve the diagnosis, treatment, and management of a vast array of health conditions all with a simple blood draw.
- Cell-Free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell. 2016 Jan 14;164(1-2):57-68.
- In the News: A New Tool in the Toolbox: New Method Traces Free-Floating DNA Back to Its Source
Using Gene Editing to Treat Duchenne Muscular Dystrophy
 Amy Wagers (2008 New Innovator), George Church (2013 Transformative Researcher), and Feng Zhang (2012 Pioneer; 2010 & 2015 Transformative Researcher) published back-to-back Science papers on using the CRISPR-Cas9 system to edit the genome of a mouse model of Duchenne muscular dystrophy and improving muscle function. The researchers loaded a virus with the DNA-cutting tool CRISPR and infected the mice's muscle cells. CRISPR excised a defective stretch of DNA, shortening the dystrophin protein and making it functional. The functional protein led to muscle improvement and increased strength in the mice and brings hope for one day using gene editing to treat human's with Duchenne Muscular Dystrophy.
Amy Wagers (2008 New Innovator), George Church (2013 Transformative Researcher), and Feng Zhang (2012 Pioneer; 2010 & 2015 Transformative Researcher) published back-to-back Science papers on using the CRISPR-Cas9 system to edit the genome of a mouse model of Duchenne muscular dystrophy and improving muscle function. The researchers loaded a virus with the DNA-cutting tool CRISPR and infected the mice's muscle cells. CRISPR excised a defective stretch of DNA, shortening the dystrophin protein and making it functional. The functional protein led to muscle improvement and increased strength in the mice and brings hope for one day using gene editing to treat human's with Duchenne Muscular Dystrophy.
- In Vivo Genome Editing Improves Muscle Function in a Mouse Model of Duchenne Muscular Dystrophy. Nelson CE et al. Science. 2016 Jan 22;351(6271):403-7.
- In Vivo Gene Editing in Dystrophic Mouse Muscle and Muscle Stem Cells. Tabebordbar M et al. Science. 2016 Jan 22;351(6271):407-11.
- In the News: Gene Editing Offers Hope for Treating Duchenne Muscular Dystrophy, Studies Find
- In the News: CRISPR Helps Heal Mice with Muscular Dystrophy
Identifying and Mapping Neuron Connections
 Andreas Tolias, a 2011 Pioneer, developed a new method for categorizing and mapping the connectivity between neuronal cell types. By combining octuple whole-cell recordings with an optimized staining technique, Tolias carried out a morphological and electrophysiological census of neuronal types in layers of mature neocortex and mapped the connectivity between over 11,000 pairs of identified neurons. The technique opens the way for studying and understanding complex brain functions.
Andreas Tolias, a 2011 Pioneer, developed a new method for categorizing and mapping the connectivity between neuronal cell types. By combining octuple whole-cell recordings with an optimized staining technique, Tolias carried out a morphological and electrophysiological census of neuronal types in layers of mature neocortex and mapped the connectivity between over 11,000 pairs of identified neurons. The technique opens the way for studying and understanding complex brain functions.
- Principles of Connectivity among Morphologically Defined Cell Types in Adult Neocortex. Jiang X et al. Science. 2015 November 27; 350(6264):aaac9462.
Rapid Diagnosis Using Enzymes Tethered to Nanoparticles
 Alexander Travis, a 2009 Pioneer, developed a platform technology to detect minute quantities of analytes. Enzymes attached to nanoparticles are used to detect biomarker molecules and convert that detection into light. The technology can be used to diagnose strokes in less than ten minutes using a drop of blood. The technology could eventually be expanded and used in point-of-care testing devices to diagnose other conditions in humans and animals, including traumatic brain injury, some forms of dementia, and even some types of cancer and heart disease.
Alexander Travis, a 2009 Pioneer, developed a platform technology to detect minute quantities of analytes. Enzymes attached to nanoparticles are used to detect biomarker molecules and convert that detection into light. The technology can be used to diagnose strokes in less than ten minutes using a drop of blood. The technology could eventually be expanded and used in point-of-care testing devices to diagnose other conditions in humans and animals, including traumatic brain injury, some forms of dementia, and even some types of cancer and heart disease.
- Use of Tethered Enzymes as a Platform Technology for Rapid Analyte Detection. Cohen R et al. PLOS ONE. 2015 November 25; 10(11):e0142326.
- In the News: New Technology Promises Fast, Accurate Stroke Diagnosis
Synthesizing Narcotics in Yeast
 Christina Smolke, a 2012 Pioneer, engineered yeast to produce select opioid compounds starting from sugar. Opioids are the primary drugs used for pain management and palliative care, but farming opium poppies for these crucial medicines remains unreliable due to weather, climate change, and pests. Smolke’s team inserted 23 genes from plants, animals, bacteria, and yeast to produce the necessary enzymes needed to convert the sugar stepwise into opioid compounds in the most complicated chemical synthesis ever undertaken in yeast. While major hurdles and issues with scale-up remain before yeast is a viable source for opioids, the work has ignited concerns of the technology being exploited for illicit purposes. However, Smolke remains committed to providing greater access to pain relief for pain victims around the world.
Christina Smolke, a 2012 Pioneer, engineered yeast to produce select opioid compounds starting from sugar. Opioids are the primary drugs used for pain management and palliative care, but farming opium poppies for these crucial medicines remains unreliable due to weather, climate change, and pests. Smolke’s team inserted 23 genes from plants, animals, bacteria, and yeast to produce the necessary enzymes needed to convert the sugar stepwise into opioid compounds in the most complicated chemical synthesis ever undertaken in yeast. While major hurdles and issues with scale-up remain before yeast is a viable source for opioids, the work has ignited concerns of the technology being exploited for illicit purposes. However, Smolke remains committed to providing greater access to pain relief for pain victims around the world.
- Complete Biosynthesis of Opioids in Yeast. Galanie S, Thodey K, Trenchard IJ, Filsinger Interrante M, and Smolke CD. Science. 2015 August 13; pii: aac9373.
- A Microbial Biomanufacturing Platform for Natural and Semisynthetic Opioids. Thodey K, Galanie S, and Smolke CD. Nat Chem Biol. 2014 October; 10:837-844.
- In the News: Narcotic Drugs Can Be Coaxed From Yeast
Anesthesia's Effects on the Brain Differ in the Elderly and in Children
 Patrick Purdon (2009 New Innovator) and Emery Brown (2012 Transformative Researcher and 2007 Pioneer) released four papers on brain activity under anesthesia in children and the elderly that address how to improve anesthesia care and advance the neuroscience of development and aging. They found anesthesia-induced brain dynamics mirrors brain development in children, where different brain wave patterns under anesthesia appear to “turn on” at particular ages, coinciding with known brain developmental milestones. A similar effect is seen in the elderly but in reverse—certain brain waves seem to fade away in a manner consistent with known patterns of brain aging. Elderly patients are much more sensitive to anesthetic drugs than current age-adjusted dosing guidelines suggest, and the elderly enter a coma-like state (associated with poor cognitive outcomes) at much lower doses than with young patients. Elderly animals emerge from anesthesia much more slowly than younger animals, but the drug methylphenidate accelerates emergence. Purdon and Brown also found commercially available EEG-based anesthetic monitors (developed for adults) seem inaccurate when applied to children and the elderly and suggest new age-specific monitoring guidelines.
Patrick Purdon (2009 New Innovator) and Emery Brown (2012 Transformative Researcher and 2007 Pioneer) released four papers on brain activity under anesthesia in children and the elderly that address how to improve anesthesia care and advance the neuroscience of development and aging. They found anesthesia-induced brain dynamics mirrors brain development in children, where different brain wave patterns under anesthesia appear to “turn on” at particular ages, coinciding with known brain developmental milestones. A similar effect is seen in the elderly but in reverse—certain brain waves seem to fade away in a manner consistent with known patterns of brain aging. Elderly patients are much more sensitive to anesthetic drugs than current age-adjusted dosing guidelines suggest, and the elderly enter a coma-like state (associated with poor cognitive outcomes) at much lower doses than with young patients. Elderly animals emerge from anesthesia much more slowly than younger animals, but the drug methylphenidate accelerates emergence. Purdon and Brown also found commercially available EEG-based anesthetic monitors (developed for adults) seem inaccurate when applied to children and the elderly and suggest new age-specific monitoring guidelines.
- Age-Dependent Electroencephalogram (EEG) Patterns During Sevoflurane General Anesthesia in Infants. Cornelissen L, Kim S-E, Purdon PL, Brown EN, and Berde CB. eLife. 2015 June 23; 4.e06513.
- The Ageing Brain: Age-Dependent Changes in the Electroencephalogram During Propofol and Sevoflurane General Anaesthesia. Purdon PL et al. Br J Anaesth. 2015 July; 115(Suppl 1):i46-i57.
- Ageing Delays Emergence from General Anaesthesia in Rats by Increasing Anaesthetic Sensitivity in the Brain. Chemali JJ et al. Br J Anaesth. 2015 July; 115(Suppl 1):i58-i65.
- Age-Dependency of Sevoflurane-Induced Electroencephalogram Dynamics in Children. Akeju O et al. Br J Anaesth. 2015 July; 115(Suppl 1):i66-i76.
Syringe-Injectable Electronics
 Charles Lieber, a 2008 Pioneer, published a paper in Nature Nanotechnology demonstrating the syringe injection of microscopic mesh electronics through needles with a diameter as small as 100 μm. Lieber successfully injected >90% of the electronics into three-dimensional structures, including synthetic structures and live rodent brains. The successful injection of electronics into artificial or natural structures could allow for continuous monitoring and manipulation of the structures.
Charles Lieber, a 2008 Pioneer, published a paper in Nature Nanotechnology demonstrating the syringe injection of microscopic mesh electronics through needles with a diameter as small as 100 μm. Lieber successfully injected >90% of the electronics into three-dimensional structures, including synthetic structures and live rodent brains. The successful injection of electronics into artificial or natural structures could allow for continuous monitoring and manipulation of the structures.
Syringe-Injectable Electronics. Liu J et al. Nat Nanotechnol. 2015 June 8; 10:629–636.
HIV Latency Confers Evolutionary Advantage
 Leor Weinberger, 2013 Pioneer and 2009 New Innovator Awardee, published back-to-back papers in Cell that demonstrate HIV latency is caused by the virus itself, rather than by the immune cells as was previously thought, and identified the protein Tat as the crucial regulator of mediating whether the virus is active or latent. This work also suggests that latency is a naturally selected tactic employed by the virus to increase infection rates. This research provides important information about how and why latency is established and maintained, opening the door to novel therapeutic approaches to treat HIV infection.
Leor Weinberger, 2013 Pioneer and 2009 New Innovator Awardee, published back-to-back papers in Cell that demonstrate HIV latency is caused by the virus itself, rather than by the immune cells as was previously thought, and identified the protein Tat as the crucial regulator of mediating whether the virus is active or latent. This work also suggests that latency is a naturally selected tactic employed by the virus to increase infection rates. This research provides important information about how and why latency is established and maintained, opening the door to novel therapeutic approaches to treat HIV infection.
A hardwired HIV latency program. Razooky BS et al. Cell. 2015 Feb 26; 160(5):990-1001.
An evolutionary role for HIV latency in enhancing viral transmission. Rouzine IM, Weinberger AD, Weinberger LS. Cell. 2015 Feb 26; 160(5):1002-1012.
Read the press release from Gladstone Institutes.
Measuring Individual Molecules
 Michael Roukes, a 2010 awardee, developed a new methodology enabling the simultaneous measurements of mass, position, molecular size, and shape of individual analytes as they adsorb onto a nanomechanical resonator. Each single-molecule adsorption event produces discrete, time-correlated perturbations. When these events are continuously monitored at different vibrational modes, characteristics of individual molecules can be measured simultaneously. This imaging technique can enable single-molecule mass spectrometry and the evaluation of the size and shape of individual molecules in the nanometric-scale.
Michael Roukes, a 2010 awardee, developed a new methodology enabling the simultaneous measurements of mass, position, molecular size, and shape of individual analytes as they adsorb onto a nanomechanical resonator. Each single-molecule adsorption event produces discrete, time-correlated perturbations. When these events are continuously monitored at different vibrational modes, characteristics of individual molecules can be measured simultaneously. This imaging technique can enable single-molecule mass spectrometry and the evaluation of the size and shape of individual molecules in the nanometric-scale.
Inertial imaging with nanomechanical systems. Hanay MS et al. Nat Nanotechnol. 2015 May; 10(4):339-344.
New Device Holds Promise for Hard-to-Reach Tumors
Pioneer awardee Dr. Joseph DeSimone has developed a device that uses electrical currents to drive  chemotherapy drugs directly into tumors, potentially revolutionizing how doctors treat some of the most challenging types of cancer. Several types of cancer, notably pancreatic cancer, can be difficult to treat with surgery because the tumor can become enmeshed with healthy tissue and blood vessels. Intravenous chemotherapy treatment may also be ineffective, because these tumors often don’t have a good enough blood supply to deliver effective concentrations of the drug. To overcome these obstacles, Dr. DeSimone and colleagues at the University of North Carolina Chapel Hill, Duke University, and North Carolina State University designed a device that can be implanted directly on the tumor or applied to the skin to precisely deliver therapeutic doses chemotherapy drugs into the tumor, with minimal amounts of the drug spreading to other places in the body. In animal models of pancreatic and breast cancer, treatment with the device slowed tumor growth or even reduced tumor size. Treatment with the device was even more effective when combined with standard intravenous chemotherapy and radiation. These results suggest that the new approach pioneered by Dr. DeSimone may one day be added to the arsenal of weapons doctors use in the fight against cancer.
chemotherapy drugs directly into tumors, potentially revolutionizing how doctors treat some of the most challenging types of cancer. Several types of cancer, notably pancreatic cancer, can be difficult to treat with surgery because the tumor can become enmeshed with healthy tissue and blood vessels. Intravenous chemotherapy treatment may also be ineffective, because these tumors often don’t have a good enough blood supply to deliver effective concentrations of the drug. To overcome these obstacles, Dr. DeSimone and colleagues at the University of North Carolina Chapel Hill, Duke University, and North Carolina State University designed a device that can be implanted directly on the tumor or applied to the skin to precisely deliver therapeutic doses chemotherapy drugs into the tumor, with minimal amounts of the drug spreading to other places in the body. In animal models of pancreatic and breast cancer, treatment with the device slowed tumor growth or even reduced tumor size. Treatment with the device was even more effective when combined with standard intravenous chemotherapy and radiation. These results suggest that the new approach pioneered by Dr. DeSimone may one day be added to the arsenal of weapons doctors use in the fight against cancer.
Read the press release from UNC Chapel Hill
Read the NBC News story "Device Reaches Where Cancer Drugs Cannot"
Reference:
Byrne et al. Local ionophoretic administration of cytotoxic therapies to solid tumors. Science Translational Medicine, Feb 2015, 7(273).
Breakthrough Technique Expands the Capabilities of Microscopes
 Microscopes allow researchers to peer into tiny cells and tissues by making the objects appear larger. However, visualizing extremely tiny cellular structures requires specialized microscopes that are very expensive, and even the most advanced microscopes are limited in how much they can magnify objects. Using a paradigm-shifting technique called expansion microscopy (ExM), Pioneer and Transformative Research Awardee Dr. Edward Boyden is taking the novel approach of actually making the samples themselves larger. Using a material commonly found in baby diapers, Dr. Boyden and colleagues at the Massachusetts Institute of Technology (MIT) are able to expand tissues to about 4.5 times their normal size, enabling visualization of tiny cellular structures and proteins with ordinary microscopes. This material, called sodium acrylate, holds proteins in place while it swells in the presence of water, resulting in minimal distortion of the sample’s structure. Using ExM, researchers could image a 3-dimensional slice of brain tissue and could zoom in to see the tiny synapse where neurons communicate with each other. By eliminating the need for costly, specialized equipment, ExM has the potential to enable many more scientists to examine structures otherwise unobservable in the cell.
Microscopes allow researchers to peer into tiny cells and tissues by making the objects appear larger. However, visualizing extremely tiny cellular structures requires specialized microscopes that are very expensive, and even the most advanced microscopes are limited in how much they can magnify objects. Using a paradigm-shifting technique called expansion microscopy (ExM), Pioneer and Transformative Research Awardee Dr. Edward Boyden is taking the novel approach of actually making the samples themselves larger. Using a material commonly found in baby diapers, Dr. Boyden and colleagues at the Massachusetts Institute of Technology (MIT) are able to expand tissues to about 4.5 times their normal size, enabling visualization of tiny cellular structures and proteins with ordinary microscopes. This material, called sodium acrylate, holds proteins in place while it swells in the presence of water, resulting in minimal distortion of the sample’s structure. Using ExM, researchers could image a 3-dimensional slice of brain tissue and could zoom in to see the tiny synapse where neurons communicate with each other. By eliminating the need for costly, specialized equipment, ExM has the potential to enable many more scientists to examine structures otherwise unobservable in the cell.
(Photo: Dr. Edward Boyden presenting ExM at the Common Fund's High Risk, High Reward Research Symposium in December 2014)
Listen to Dr. Boyden’s talk at the Common Fund’s High Risk, High Reward Research Symposium (starts at 1 hour, 33 minutes)
Read the press release from MIT
Reference:
Chen F, Tillberg PW, and Boyden ES. Expansion Microscopy. Science, Jan 2015, DOI: 10.1126/science.1260088.
REST: The Difference between Destruction and Protection of the Brain
The maintenance of cognitive ability during the aging process has become a significant medical challenge of our time. Alzheimer’s disease (AD), currently having no treatment, is one of the leading causes of death in the United States. A host of other neurodegenerative diseases cause a decline in mental ability that is capable of interfering with daily life. The reasons for the onset of these diseases are still being examined. Earlier studies suggest that neuronal loss was a normal consequence of brain aging; however, neuronal cells are preserved in the aging brain and decline only in the presence of neurodegenerative disease. Much of the research into the causes of diseases resulting in dementia has focused on the abnormal proteins that appear in the brains of people with neurodegenerative diseases; however some people with these abnormal protein clumps show little or no signs of cognitive decline. Why is it that some individuals, even those presenting dementia pathology, age with their cognitive function intact while others develop dementia?
A new study, led by Pioneer awardee, Dr. Bruce Yankner of Harvard Medical School, begins to answer this question. Dr. Yankner and colleagues have discovered a mechanism involving repressor element 1-silencing transcription factor (REST), a repressor of neuronal genes during embryonic development that is down regulated after this stage. The researchers show that REST returns later in life to protect aging neurons from various stresses and regulates a network of genes that mediate cell death, stress resistance and dementia pathology. In an example involving AD, in its early stages, REST disappears from the nucleus, causing the gene network to lose regulation. Further experiments in the study indicate that REST protects neurons from age-related toxic insults and that the elevated REST levels in aging humans are associated with the preservation of cognitive function as well as increased longevity. Additionally, the presence of REST increases the expression of other transcription factors and enzymes that act to resist oxidative stress. REST is also shown to protect against a model of a different dementia disease, Parkinson’s disease, indicating that it might be involved in a stress response that is protective of neurons in a range of dementia diseases. So, even when harboring the abnormal protein clumps associated with dementia diseases, when neuronal REST levels are high, these individuals do not proceed to dementia, suggesting that dementia protein presence may not be enough and that failure of the brain’s stress response system may also be necessary to present cognitive inability. This finding introduces new possibilities for therapeutic intervention.
To read more about this research, please see the publication in Nature.
Please also see the related press release from Dr. Yankner’s institution, Harvard Medical School.
Two NIH Common Fund High Risk – High Reward Researchers named as 2013 Allen Distinguished Investigators
The Paul G. Allen Family Foundation, co-founded by Paul G. Allen (Microsoft co-founder) and his sister, Jo Lynn Allen, with a goal of “transforming lives and strengthening communities by fostering innovation, creating knowledge, and promoting social progress” through its philanthropic efforts. In 2010 the Foundation created the Allen Distinguished Investigators Awards to support high-risk, high-reward ideas in science. The program awards grants to ambitious scientists, who are pioneering innovative early-stage research. Two NIH High Risk – High Reward Investigators, Jeff Gore, a 2012 New Innovator from MIT, and Markus Covert, a 2009 Pioneer from Stanford University, were selected as 2013 Allen Distinguished Investigators. Dr. Gore will use the $1.5 million prize to investigate decision making and the evolutionary origins or cooperation using yeast as a model. Dr. Covert will also receive $1.5 million and plans to use this grant to study whole-cell models of higher organisms, including human cells. These awards will be funded over the course of three years and the Foundation hopes these grants will enable the awardees to unlock fundamental questions in biology that are essential to achieving world-changing breakthroughs.
Read more about the Allen Distinguished Investigators Award here ,
and read the Paul G. Allen Family Foundation Press Release for the 2013 Allen Distinguished Investigators here.
Two HRHR researchers awarded world’s largest brain research prize
Two NIH Common Fund High Risk-High Reward Awardees were named as winners of The Brain Prize for 2013, which is awarded by the Grete Lundbeck European Brain Research Foundation. Americans, Karl Deisseroth, a 2005 Pioneer Awardee, and 2012 Transformative Research Awardee, and Edward S. Boyden, a 2007 New Innovator, and 2012 Transformative Research Awardee and four European scientists were awarded the 2013 prize for their contributions to the development of “optogenetics.” Optogenetics is a technique that merges the fields of optics and genetics using light to specifically control the activity of genetically selected neurons. This revolutionary technique has allowed scientists to begin to gain a better understanding of the way circuits of neurons carry out complex functions. This technique has broad application potential in research from studying fundamental activities, such as memory formation and breathing, to more complex disorders and diseases, such as addiction, psychiatric disorders, and Alzheimer’s disease.
The Brain Prize, the world’s largest brain research prize, is awarded to scientists who have made an outstanding contribution to European brain research, and is awarded to scientists who have conducted research in Europe, or to scientists who have conducted research in collaboration with European scientists.
Read more about the Brain Prize here , and read the 2013 Brain Prize press release here.
Rats Communicate Via Brain-To-Brain Interface
Miguel A. L. Nicolelis, a 2010 NIH Director’s Pioneer Awardee from Duke University, and his colleagues, have connected the brains of rat pairs through the internet and shown that the rats can share sensory information, acting as a dyad rather than strictly as individuals. Dr. Nicolelis’ lab is well known for their work on brain-to-machine interface (BTM), the current paradigm used to help develop technology that allows an amputee’s brain to control a prosthetic limb. The team of researchers took the brain interfacing concept further and connected pairs of rat brains using a new paradigm they termed brain-to-brain interface (BTBI). This BTBI enabled the rat pairs to act as a dyad exchanging sensorimotor information to achieve simple behavioral goals. The researchers implanted microelectrode bundles in the cortical area in the brains of rats. These microelectrodes allowed the researchers to record the neural activity of an “encoder” rat acting out a specific behavior and directly transmit this recorded signal to a “decoder” rat’s brain using intracortical microstimulation (ICMS), the stimulation of individual nerve cells using a small electric current, to inform the decoder rat to complete a specific activity.
The encoder rat was trained to press one of two levers in response to a light stimulus above one lever. While the encoder rat pressed this lever, the neural activity was recorded and transformed into ICMS which was then applied to the brain of the decoder rat informing the decoder rat of which lever to press. If the decoder rat pressed the correct lever, both rats received a reward. If the decoder rat pressed the incorrect lever, neither rat received a reward. The researchers took this experiment a step further and were able to perform this experiment in real time, with the encoder rat located in Natal, Brazil, and the decoder rat located at Duke University in North Carolina, transmitting the ICMS over the internet. In a second set of experiments, the scientists trained the encoder rat to determine the width of an opening (wide or narrow) using its whiskers and to push either the right or left lever, respectively. Again, the neural activity of the encoder rat’s brain during this task was recorded and transmitted by ICMS to the decoder rat informing the decoder of which lever to press. In both activities, the decoder rats pressed the correct lever significantly more often than rats who were not receiving neural activity information via ICMS. Further, the encoder rat changed both its behavior and its neural signals in response to feedback from the decoder rat’s behavior, indicating that the rat pairs were acting as dyads indicating a more complex system of communication. The researchers also mechanically stimulated both the encoder and decoder rat’s whiskers and recorded the neural response to this stimulation. They then stimulated the encoder rat’s whiskers, recorded the activity and sent it the decoder rat’s brain using ICMS and recorded the neural activity of the decoder rats brain in response. Passive whisker stimulation in either the encoder or decoder rats induced significant neural activity in the decoder rat’s brain.
Through this set of experiments the researchers demonstrated that tactile and motor information can be recorded in real-time from a rat’s brains and transmitted directly into another rat’s brain using BTBI. In this BTBI rat dyad, the decoder rat relied exclusively on the neural patterns generated by the encoder rat in order to reproduce the encoder rat’s behavioral choice. The ICMS patterns reflecting the neural activity in the encoder rat’s brain was sufficient to inform decoder rats to perform both tactile and motor activities significantly above chance in real-time. The potential impact of this research is not limited to behavioral information exchange of animal dyads, but could be expanded to create a multi-brain system where groups of interconnected brain networks could communicate through large scale BTBI.
Read the Duke University Press Release here
Reference:
M. Pais-Vieira, M. Lebedev, C. Kunicki, J. Wang, M. A. L. Nicolelis, A Brain-to-Brain Interface for Real-Time Sharing of Sensorimotor Information. Sci. Rep. 3, (2013). PMID: 23448946
Researchers define precise EEG Signature of Anesthesia-Induced Unconsciousness
 More than 60,000 patients in the United States undergo general anesthesia for surgery every day. Currently, doctors use indirect measures of the brain state, such as heart rate and blood pressure, along with drug pharmacokinetics, pharmacodynamics and exhaled anesthetic gas typically are converted to a simple index score to indicate if a patient is adequately anesthetized during surgery. These indices are neither entirely reliable nor very informative. Inadequately administered anesthesia can result in intraoperative awareness and post-operative delirium. Drs. Emery N. Brown and Patrick L. Purdon, former NIH Director’s Pioneer Awardee and current NIH Director’s New Innovator Awardee, respectively, have studied brain activity during propofol-induced anesthesia using electroencephalogram (EEG) technology, which measures scalp electrical potentials, to better understand the mechanism of unconsciousness and to establish a direct method of tracking the transition between consciousness and unconsciousness during general anesthesia. In this study, the researchers gradually administered and reduced the anesthesia drug propofol in patients, while monitoring brain activity and behavioral loss of consciousness by asking patients to respond to auditory stimuli. This gradual induction and emergence from anesthesia allowed the researchers to precisely define an EEG brain signature, as well as behavioral markers that are associated with consciousness, unconsciousness, and the transition between these two states in patients undergoing propofol-induced general anesthesia. This research has provided a deeper understanding of the mechanism of propofol-induced loss of consciousness, and could be used to determine the EEG brain signatures of other anesthetics. Additionally, these results could be used to develop more direct and reliable methods for monitoring brain states of patients undergoing general anesthesia and could lead to new insights into the tailoring of drug dosage in real-time.
More than 60,000 patients in the United States undergo general anesthesia for surgery every day. Currently, doctors use indirect measures of the brain state, such as heart rate and blood pressure, along with drug pharmacokinetics, pharmacodynamics and exhaled anesthetic gas typically are converted to a simple index score to indicate if a patient is adequately anesthetized during surgery. These indices are neither entirely reliable nor very informative. Inadequately administered anesthesia can result in intraoperative awareness and post-operative delirium. Drs. Emery N. Brown and Patrick L. Purdon, former NIH Director’s Pioneer Awardee and current NIH Director’s New Innovator Awardee, respectively, have studied brain activity during propofol-induced anesthesia using electroencephalogram (EEG) technology, which measures scalp electrical potentials, to better understand the mechanism of unconsciousness and to establish a direct method of tracking the transition between consciousness and unconsciousness during general anesthesia. In this study, the researchers gradually administered and reduced the anesthesia drug propofol in patients, while monitoring brain activity and behavioral loss of consciousness by asking patients to respond to auditory stimuli. This gradual induction and emergence from anesthesia allowed the researchers to precisely define an EEG brain signature, as well as behavioral markers that are associated with consciousness, unconsciousness, and the transition between these two states in patients undergoing propofol-induced general anesthesia. This research has provided a deeper understanding of the mechanism of propofol-induced loss of consciousness, and could be used to determine the EEG brain signatures of other anesthetics. Additionally, these results could be used to develop more direct and reliable methods for monitoring brain states of patients undergoing general anesthesia and could lead to new insights into the tailoring of drug dosage in real-time.
Reference:
P. L. Purdon et al., Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proceedings of the National Academy of Sciences, (March 4, 2013, 2013).
Scientists Uncover How Tuberculosis “Pumps-Up” Tolerance To Drug Therapies
 Shortening the time required to treat tuberculosis (TB) is key to reducing the development of drug resistance and lowering worldwide rates of TB infection and mortality. Reducing treatment duration however depends on: (1) understanding how Mycobacterium tuberculosis (Mtb), the bacteria that causes TB in humans, becomes tolerant to antibiotics; and (2) devising ways to prevent or overcome drug tolerance. NIH Director’s Pioneer Award recipient Dr. Lalita Ramakrishnan and colleagues at the University of Washington, in collaboration with Dr. Paul Edelstein at the University of Pennsylvania, report in a study appearing in the April 1, 2011 issue of Cell, that the development of drug tolerance in Mtb is due in part to the activity of “efflux” pumps in the cell membrane that presumably flush away any antibiotic agents that penetrate the Mtb cells. The authors found that these pumps are stoked into action by host cells called macrophages that are, ironically, part of the body’s frontline defenses against foreign invaders. Based on these findings, the study authors suggest that expanding treatment to include medications that inhibit these pumps could dramatically shorten the time needed to cure infection. While Mtb can infect many body tissues and organs, it primarily attacks the lungs. Once inside the lungs, the bacteria infect the aforementioned macrophages. The macrophages and other types of immune cells respond by aggregating into structures called granulomas which are thought to contain the spread of persistent pathogens. Having taken up residence within macrophages, some populations of Mtb cells quickly become tolerant to anti-TB drugs. Nearly all models of Mtb drug tolerance postulate that this temporary resistance to antibiotics arises when the bacterial cells enter a dormant state in which they stop replicating. Because most antibiotics are only effective against bacterial cells that are reproducing, dormant cells are effectively resistant to antimicrobial agents. In their latest paper however, Dr. Ramakrishnan and colleagues report finding multi-drug tolerant Mtb populations that were actively growing and reproducing inside of host macrophages suggesting that residence within macrophages rapidly induces tolerance. The study authors also found that, having infected macrophages, the Mtb cells deploy efflux pumps that are essential for the Mtb cells to grow within the macrophages and may be used by the bacterial cells to remove toxic substances such as antimicrobial agents. In addition to expanding our understanding of the pathogenesis of TB and drug tolerance, these findings suggest that inhibiting the activity of macrophage-induced bacterial efflux pumps using currently available drugs such as verapamil may be an effective means of reducing the duration of TB treatment. Shorter treatment is likely to translate into increased adherence which will in turn slow the development of multi-drug resistance, reduce transmission of infection to new hosts, and reduce TB-associated mortality around the world.
Shortening the time required to treat tuberculosis (TB) is key to reducing the development of drug resistance and lowering worldwide rates of TB infection and mortality. Reducing treatment duration however depends on: (1) understanding how Mycobacterium tuberculosis (Mtb), the bacteria that causes TB in humans, becomes tolerant to antibiotics; and (2) devising ways to prevent or overcome drug tolerance. NIH Director’s Pioneer Award recipient Dr. Lalita Ramakrishnan and colleagues at the University of Washington, in collaboration with Dr. Paul Edelstein at the University of Pennsylvania, report in a study appearing in the April 1, 2011 issue of Cell, that the development of drug tolerance in Mtb is due in part to the activity of “efflux” pumps in the cell membrane that presumably flush away any antibiotic agents that penetrate the Mtb cells. The authors found that these pumps are stoked into action by host cells called macrophages that are, ironically, part of the body’s frontline defenses against foreign invaders. Based on these findings, the study authors suggest that expanding treatment to include medications that inhibit these pumps could dramatically shorten the time needed to cure infection. While Mtb can infect many body tissues and organs, it primarily attacks the lungs. Once inside the lungs, the bacteria infect the aforementioned macrophages. The macrophages and other types of immune cells respond by aggregating into structures called granulomas which are thought to contain the spread of persistent pathogens. Having taken up residence within macrophages, some populations of Mtb cells quickly become tolerant to anti-TB drugs. Nearly all models of Mtb drug tolerance postulate that this temporary resistance to antibiotics arises when the bacterial cells enter a dormant state in which they stop replicating. Because most antibiotics are only effective against bacterial cells that are reproducing, dormant cells are effectively resistant to antimicrobial agents. In their latest paper however, Dr. Ramakrishnan and colleagues report finding multi-drug tolerant Mtb populations that were actively growing and reproducing inside of host macrophages suggesting that residence within macrophages rapidly induces tolerance. The study authors also found that, having infected macrophages, the Mtb cells deploy efflux pumps that are essential for the Mtb cells to grow within the macrophages and may be used by the bacterial cells to remove toxic substances such as antimicrobial agents. In addition to expanding our understanding of the pathogenesis of TB and drug tolerance, these findings suggest that inhibiting the activity of macrophage-induced bacterial efflux pumps using currently available drugs such as verapamil may be an effective means of reducing the duration of TB treatment. Shorter treatment is likely to translate into increased adherence which will in turn slow the development of multi-drug resistance, reduce transmission of infection to new hosts, and reduce TB-associated mortality around the world.
Reference:
Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell, 2011 April 1; 145(1): 39-53. PMID: 21376383.
Novel Blood Test May Improve Diagnosis of Many Diseases
While simple blood tests can accurately and efficiently screen for some diseases, such as diabetes, the lack of blood tests for the majority of diseases can result in delayed or incorrect diagnoses. To overcome this diagnostic obstacle, Dr. Thomas Kodadek, a researcher at The Scripps Research Institute and funded in part by an NIH Director’s Pioneer Award, has developed a novel screening method that could detect disease-associated proteins in the blood of patients with a variety of conditions. In the January 7, 2011 edition of the journal Cell, Dr. Kodadek and colleagues describe their technique for using a collection of synthetic molecules to detect the presence of unique proteins in the blood of diseased individuals that do not appear in the blood of healthy individuals. By screening blood samples from mice with multiple sclerosis, the researchers identified several molecules in their synthetic collection that would only bind to proteins in the blood of the diseased mice, and could distinguish between “patient” mice and normal, healthy mice. Importantly, the researchers went on to demonstrate that in samples from humans, they could use the same technique to identify different molecules that bound to proteins uniquely present in the blood of patients with Alzheimer’s disease. This same binding did not occur in samples from healthy people of similar ages or in patients with another neurodegenerative disorder, Parkinson’s disease. These promising results suggest that this type of blood test has the potential to screen for a wide variety of different diseases, including diseases that currently lack a reliable diagnostic test.
Reference:
Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of candidate IgG biomarkers for Alzheimer’s disease via combinatorial library screening. Cell, 2011 Jan 7; 144(1): 132-42. PMID: 21215375. September 13, 2010
Gut Microbes Changed by Repeated Antibiotic Use
 Repeated use of the antibiotic ciprofloxacin (Cipro) leads to persistent changes in the beneficial microbes of the gut, according to a study by David Relman, a researcher at Stanford University and recipient of an NIH Director's Pioneer Award. While ciprofloxacin usually does not cause gastrointestinal side effects normally associated with disturbance of gut-dwelling bacteria, this research demonstrates the occurrence of more subtle changes in gut microbe composition, such as replacement of some bacterial species with closely related species or eradication of some sub-sets of bacteria, particularly when multiple courses of antibiotics are administered. These long-term, persistent changes in microbe composition raise concerns about the evolution of antibiotic-resistant bacteria as well as chronic changes in pathogen-host interactions in the gut, regulation of host immunity, energy balance, or metabolism.
Repeated use of the antibiotic ciprofloxacin (Cipro) leads to persistent changes in the beneficial microbes of the gut, according to a study by David Relman, a researcher at Stanford University and recipient of an NIH Director's Pioneer Award. While ciprofloxacin usually does not cause gastrointestinal side effects normally associated with disturbance of gut-dwelling bacteria, this research demonstrates the occurrence of more subtle changes in gut microbe composition, such as replacement of some bacterial species with closely related species or eradication of some sub-sets of bacteria, particularly when multiple courses of antibiotics are administered. These long-term, persistent changes in microbe composition raise concerns about the evolution of antibiotic-resistant bacteria as well as chronic changes in pathogen-host interactions in the gut, regulation of host immunity, energy balance, or metabolism.
Reference:
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences, 2011 Mar 15; 108 Suppl 1: 4554-61. Epub 2010 Sep 16. PMID: 20847294.
Scientists Uncover New Process Regulating Electrical Firing Properties of Neurons
 Neurons in the brain communicate with each other using a combination of carefully regulated chemical and electrical signals. NIH Director’s Pioneer Award recipient Dr. James Eberwine and colleagues at the University of Pennsylvania and Sequenom, Inc. have discovered a novel method by which neurons selectively express proteins that control their electrical properties , reported in the December 7, 2010 issue of the Proceedings of the National Academy of Sciences. In neurons, electrical signals are controlled by channel proteins that create a pore in the cell membrane to allow positively or negatively charged ions to flow in and out of the cell. One of these channel proteins, called BKCa, helps regulate electrical signals in the hippocampus, an area of the brain that is important for learning and memory. Interestingly, the hippocampus is also the focus of many epileptic seizures, which result from disturbances in the electrical firing of neurons. The BKCa channel protein has several different variations. These protein variations arise during a cellular process called “splicing,” which is analogous to the process of cutting an undesired segment out of an audiotape and joining the resulting ends together. To make a protein, a cell must identify the piece of DNA that contains the instructions for the protein (called a gene), copy the DNA into another form of genetic material, called RNA, and then use the information encoded in the RNA to make the protein. Often, the instructions in the DNA for making a protein are not in a continuous stretch, but contain intervening sequences or “introns.” After the DNA is copied into RNA, these introns are removed and the flanking coding sequences, or “exons,” are connected to each other. It is the sequence of merged exons that tells the cell how to make a protein. Since the RNA can be spliced in different ways, one gene sequence can be used to make several different protein variants, depending on which exons are included. Normally splicing takes place in the nucleus of a cell, the cell’s control center where the DNA is stored. However, previous work from Dr. Eberwine’s lab has shown that splicing can also occur outside the nucleus, in a neuron’s cytoplasm. Dr. Eberwine’s latest paper demonstrates for the first time that one of the introns removed during cytoplamic splicing can regulate which form of BKCa channel protein is made, which in turn affects important electrical properties of the neuron. While introns used to be considered “junk DNA,” a number of studies, including Dr. Eberwine’s papers, are demonstrating that introns play crucial regulatory roles. Studying the process of splicing and intron removal in the production of BKCa channels may provide new insights about disorders of neuronal misfiring, such as epilepsy.
Neurons in the brain communicate with each other using a combination of carefully regulated chemical and electrical signals. NIH Director’s Pioneer Award recipient Dr. James Eberwine and colleagues at the University of Pennsylvania and Sequenom, Inc. have discovered a novel method by which neurons selectively express proteins that control their electrical properties , reported in the December 7, 2010 issue of the Proceedings of the National Academy of Sciences. In neurons, electrical signals are controlled by channel proteins that create a pore in the cell membrane to allow positively or negatively charged ions to flow in and out of the cell. One of these channel proteins, called BKCa, helps regulate electrical signals in the hippocampus, an area of the brain that is important for learning and memory. Interestingly, the hippocampus is also the focus of many epileptic seizures, which result from disturbances in the electrical firing of neurons. The BKCa channel protein has several different variations. These protein variations arise during a cellular process called “splicing,” which is analogous to the process of cutting an undesired segment out of an audiotape and joining the resulting ends together. To make a protein, a cell must identify the piece of DNA that contains the instructions for the protein (called a gene), copy the DNA into another form of genetic material, called RNA, and then use the information encoded in the RNA to make the protein. Often, the instructions in the DNA for making a protein are not in a continuous stretch, but contain intervening sequences or “introns.” After the DNA is copied into RNA, these introns are removed and the flanking coding sequences, or “exons,” are connected to each other. It is the sequence of merged exons that tells the cell how to make a protein. Since the RNA can be spliced in different ways, one gene sequence can be used to make several different protein variants, depending on which exons are included. Normally splicing takes place in the nucleus of a cell, the cell’s control center where the DNA is stored. However, previous work from Dr. Eberwine’s lab has shown that splicing can also occur outside the nucleus, in a neuron’s cytoplasm. Dr. Eberwine’s latest paper demonstrates for the first time that one of the introns removed during cytoplamic splicing can regulate which form of BKCa channel protein is made, which in turn affects important electrical properties of the neuron. While introns used to be considered “junk DNA,” a number of studies, including Dr. Eberwine’s papers, are demonstrating that introns play crucial regulatory roles. Studying the process of splicing and intron removal in the production of BKCa channels may provide new insights about disorders of neuronal misfiring, such as epilepsy.
Reference:
Bell TJ, Miyashiro KY, Sul JY, Buckley PT, Lee MT, McCullough R, Jochems J, Kim J, Cantor CR, Parsons TD, Eberwine JH. Intron retention facilitates splice variant diversity in calcium-activated big potassium channel populations. Proceedings of the National Academy of Sciences, 2010 Dec 7; 107(49): 21152-7. PMID: 21078998.